|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
THE DESIGN OF REINFORCED CEMENT-BASED PROTECTIVE COATINGS BY PAUL BENNISON, B.Sc., C.Eng., M.I.C.E., M.I.Struct.E., F.F.B. JOINT MANAGING DIRECTOR FLEXCRETE LIMITED ASTRAL HOUSE P.O. BOX 7 MILLER STREET PRESTON LANCASHIRE PR1 1EA
INTRODUCTION This paper describes the design of cement-based coatings which have a high diffusion resistance to gases such as Carbon Dioxide and Oxygen, as well as Chloride Ions. They are also impermeable to water molecules even under a 10 bar pressure, although they will allow the diffusion of vapour. By reinforcing with a variety of fibres and meshes, tough protective membranes can be formed with all the similarities to concrete. The incorporation of Migratory Corrosion Inhibitors also enhances their abilities to retard the corrosion mechanisms of the steel reinforcement by providing a protective film having diffused through the pore system in the vapour phase.
MATERIALS USED IN CEMENTITIOUS COATINGS
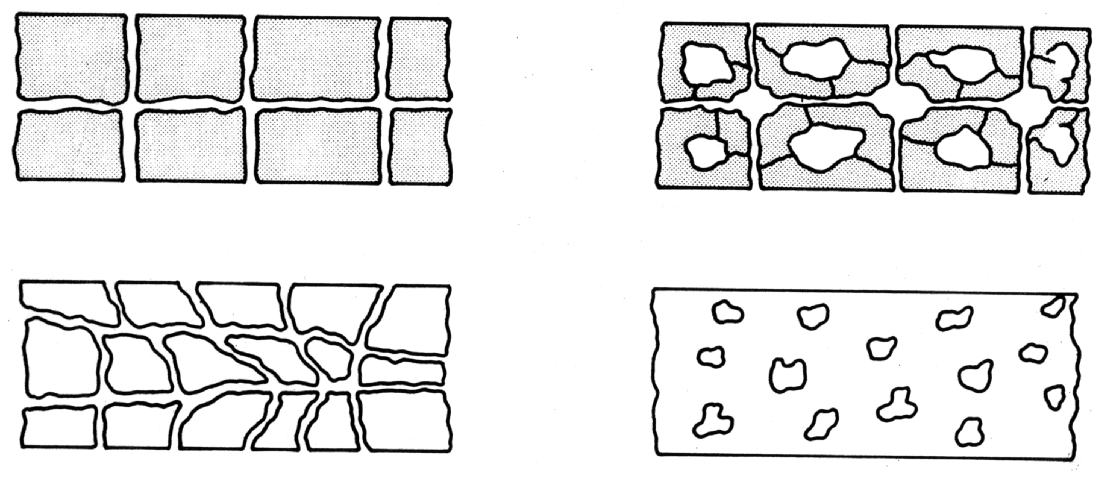
DURABILITY OF CEMENT BASED PRODUCTS The following factors will significantly affect the durability of cement based products:- 1. The mix design. 2. The cement content and type. 3. The water/cement ratio. 4. The degree and type of compaction. 5. The quality and type of aggregates. 6. Curing. 7. The use of chemical admixtures. 8. The use of polymers. 9. The use of pozzolanic materials. POROSITY AND PERMEABILITY For a cement matrix having a coherent pore system (porosity) to become permeable, there must be some form of interconnecting system of tubes or canals. Obviously, discrete sealed pores will not lead to either air or water permeability (1) (see Fig. 1). Also the size and width of pores have an influence on permeability, because the narrower the pores, the higher must be the pressure to force a liquid (generally water) through the pore system. It is important, therefore, to distinguish between air pores, capillary pores and gel pores. Small air pores are usually artificially entrained into the matrix by chemical admixtures in order to increase workability and enhance resistance to the effects of frost. Large, irregular air pores are caused by poor placing and compaction of concrete. Capillary and gel pores are as a result of the hydration process of cement and water.
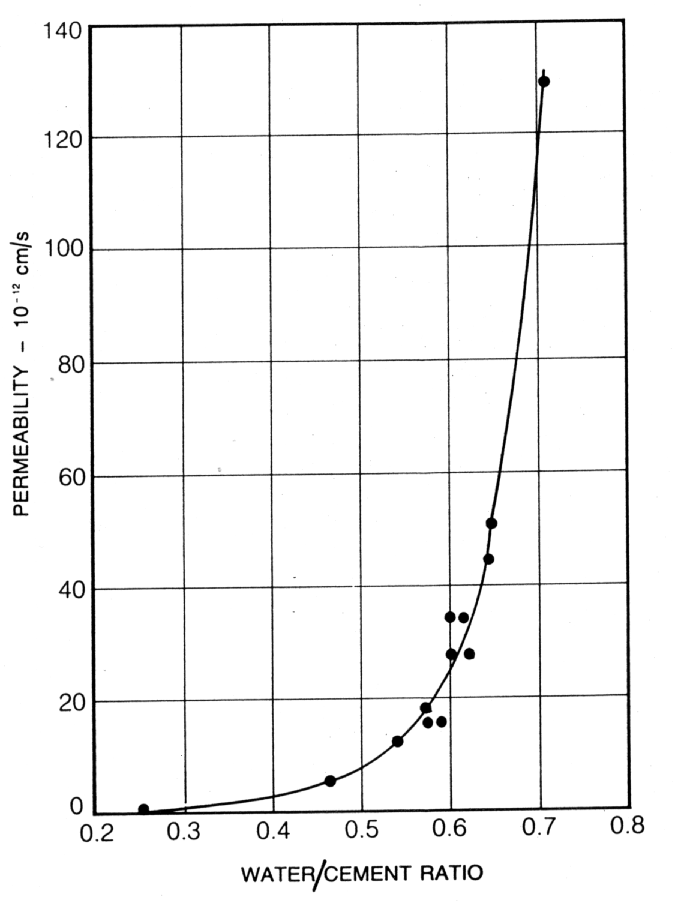
In fact, the porosity and permeability of the hardened cement paste depends upon the water/cement ratio. There is, therefore, a relationship between water/cement ratio and compressive strength (2), capillary porosity and compressive strength (3), and permeability and water/cement ratio (4) (see Figs. 2, 3 and 4). It is, however, important to note that, while strength and diffusion are affected by porosity, they are not uniquely inter-related. Thus, the nature of the hardened cement can be summarised as follows:- Immediately after mixing the cement and water together, an agglomeration of particles and water is formed. The water in the interconnecting cavities is termed "capillary water". Until it has been completely hydrated, Portland cement chemically binds water equivalent to approximately a quarter of its weight. Therefore, the water loses approximately a quarter of its volume. In addition to this chemically bound water, Portland cement loosely binds approximately 15% of its weight as "gel water". Despite its loose chemical bond, gel water is not able to react with unhydrated cement and, in fact, evaporates in dry air or in an oven at 105°C. It is this gel water and the additional water required to "lubricate" the mix to form a workable material, that create more capillaries as they become evaporable water. Therefore, the greater the water/cement ratio, the greater the permeability (see Fig. 4). The hydration by-products of the cement/water reaction form a coherent, homogeneous mass, the "cement gel". The cement gel is, however, made up of approximately 25% by volume of finely distributed pores, namely "gel pores". The total porosity (large capillary and, to a lesser degree, gel pores) of the hardened cement paste, therefore determines its strength (5) (see Fig. 5). Fig. 1 Illustration of Permeability and Porosity
Fig. 2 Typical Relationship between Cube Strength between Cube Strength Fig. 3 Typical Relationship and Water/Cement Ratio and Capillary Porosity
CEMENT CONSTITUENTS PROPERTIES AFFECTED C3S Tricalcium Silicate 55% Strength up to 28 days/setting time. C2S Dicalcium Silicate 20% Long term strength, i.e. after 28 days. C3A Tricalcium Aluminate 10% Setting time/24 hours strength. C4AF Tetracalcium Aluminoferrite 8% Very little effect.
MAIN HYDRATION PRODUCTS C-S-H Calcium Silicate Hydrates : Form the "gel" structure. C3AH6 Calcium Aluminate Hydrate. Ca(OH)2 Calcium Hydroxide : Gives the cement paste its high alkalinity HEAT Permeability and porosity can, therefore, be influenced in several ways, i.e.:- 1. The use of chemical admixtures a) To lower numerically the water/cement ratio and reduce the amount of water in the mix;
2. Curing A grain of cement will only hydrate when it is totally surrounded by a membrane of water. If this membrane is ruptured by evaporation, then the hydration process is adversely affected. The effects of poor curing manifest themselves in durability of the concrete in terms of possibly surface drying, causing plastic shrinkage cracking and, also, unhydrated cement, which may cause further disruption when re-hydrolysed. Fig. 4 Relationship between Permeability and Water/Cement Ratio for Mature Cement Pastes
Fig. 5 Hydration of Cement
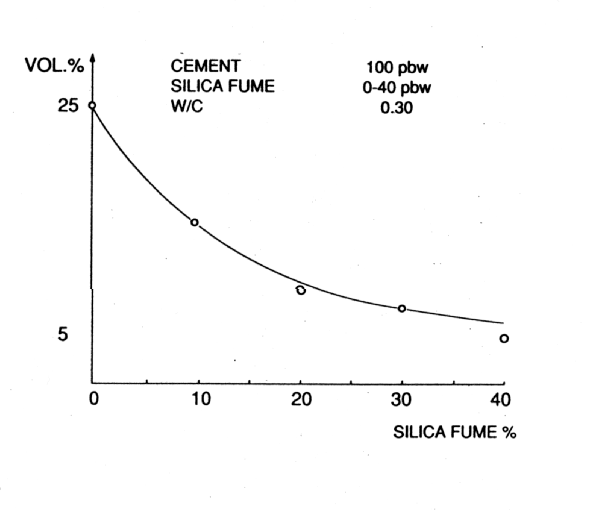
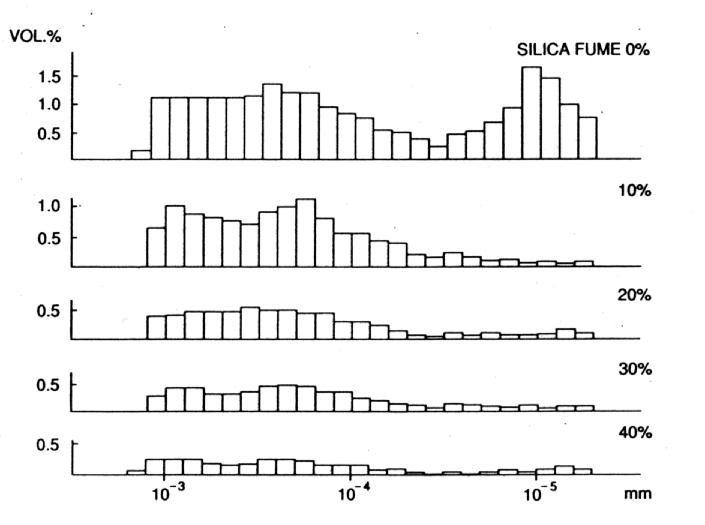
MATERIALS WHICH INFLUENCE POROSITY AND PERMEABILITY IN CEMENT PASTES "POROSITY" is defined as the total volume fraction of hollow spaces (i.e. pores) in a material. A fluid cannot pass through the material if the pores are discrete. "PERMEABILITY" is defined as the rate of flow of a fluid under a pressure differential through a material with inter-connected pores. Porosity Permeability * Microsilica * Fibres * Soluble Silicates * Long chain Polymers * Pulverised Fuel Ash * Low water/cement ratio * Polymers * Polymers * Ground Granulated Blast Furnace Slag * Metakaolin Fig. 6 Influence of Microsilica on Porosity of Cement Pastes - Total Pore Volume
Fig. 7 Influence of Microsilica on Porosity of Cement Pastes - Pore Size Distribution
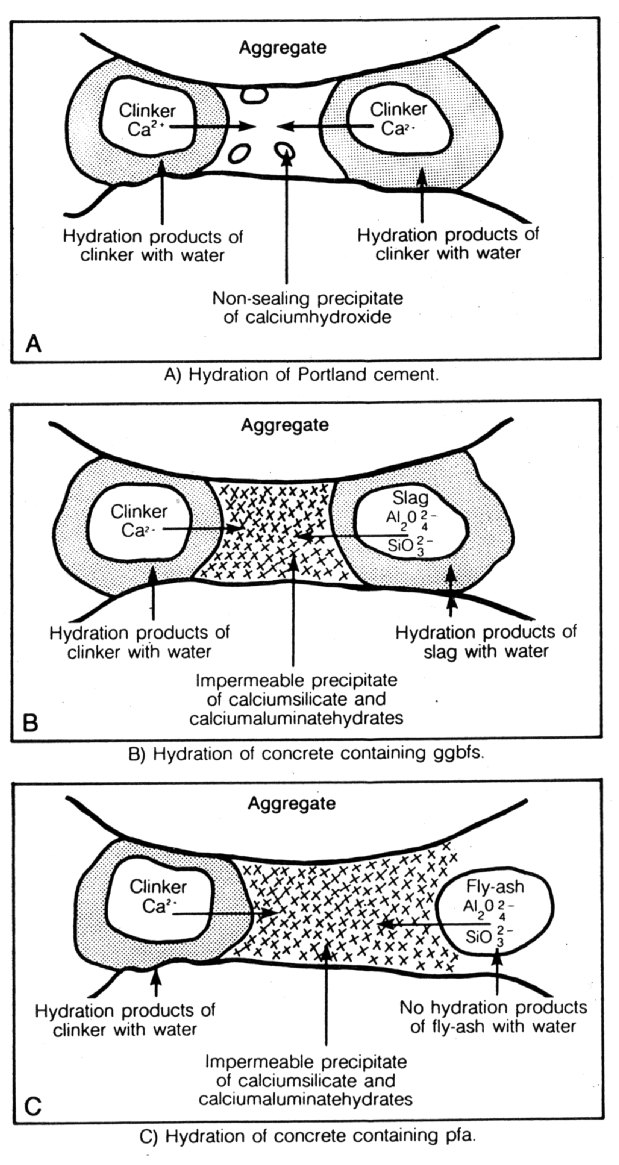
Fig. 8 Hydration of Portland Cement and Portland Cement Modified with Pozzolanic Materials
POZZOLANIC MATERIALS As stated earlier, pozzolanic materials have been used for construction since ancient times. Natural pozzolans, or in some cases calcined earths, were blended with lime (calcium hydroxide) and water to make strong cementitious materials (8). A pozzolan may be defined as a siliceous or siliceous and aluminous material which, in itself, possesses little or no cementitious value but which will, in finely divided form in the presence of moisture, react chemically with calcium hydroxide at ordinary temperature to form compounds possessing cementitious properties. Microsilica is a particularly interesting material, which can pore block (it has a mean particle size of 0.1m m) in its own right but, more importantly, by reacting with the dissolved calcium hydroxide liberated by the cement hydration, it forms stable calcium silicate hydrates (gel) which further reduce and refine the pore capillary system (see Figs. 6 and 7). It is the by-product of the manufacture of silicon and ferro silicon alloys. These processes require large quantities of electrical energy for the furnaces and, therefore, tend to be situated in countries with cheaper, hydro-generated power, i.e. Norway, Iceland and Canada. High purity quartz is introduced into the furnace and, at these very high temperatures (2000¡ C), vaporises to form silicon monoxide. This then oxidises above the furnace and condenses to form microspheres of amorphous silica. This almost pure (85-92%) silicon dioxide, in the form of a fume, is then collected in bag filters. Pulverised fuel ash is collected in a different way to that of microsilica and is a waste ash in the flue gases of coal burning power stations. The flue gases enter very large concrete precipitators horizontally, at a temperature of approximately 160¡ C. The hot gases then pass across large, vertical steel plates, which have a high voltage alternately switched across them. The charge attracts ash from the gas and the gas exits the precipitator at the other side, at a temperature of approximately 60¡ C. Once the charge has been switched off, the ash falls into a holding hopper below the precipitator. This is then usually air graded to give the acceptable particle sizes. Pulverised Fuel Ash has a similar action in cement pastes to that of microsilica, in that it produces stable calcium silicate and aluminium hydrates (see Fig. 8). However, materials produced from calcined china clays are more reactive than the above (see Fig. 9). These materials are manufactured from selected kaolins which are refined and calcined under conditions designed to give optimum pozzolanic reactivity, and are subject to strict quality control procedures. By contrast, as stated above, most other pozzolanic materials used in concrete are by-products from other industrial processes, and are well known to be highly variable in chemical composition. Fig.9 Reactivity of Pozzolanic Materials (Chappelle Test)
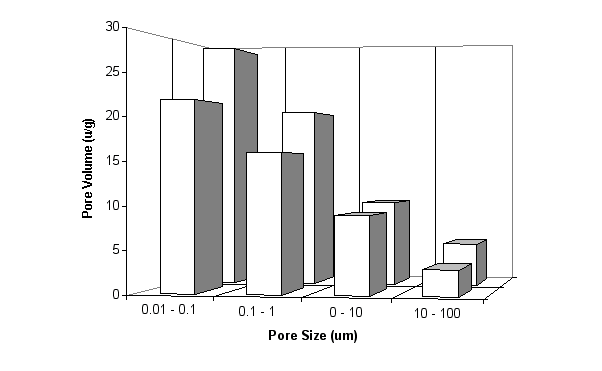
Calcined china clays react rapidly with calcium hydroxide, converting portlandite to stable cementitious compounds. At the same time they significantly reduce the porosity and permeability of the cement hydrates and the concentration of alkali metal ions (K+ and Na+) in the pore water. As can be seen from the pore size distribution from mercury intrusion porosimetry in Fig. 10, the total pore volume decreases significantly when 10% of cement is replaced by a calcined china clay based on metakaolin. Fig. 10 Effect of Metakaolin on Pore Size Distribution of a Mortar Bar
Fig. 11 Physical Data for Cementitious Materials
One of the by-products of cement hydration is heat. This can micro-crack the paste, forming continuities in the pore structure, thus increasing permeability. By acting as randomly orientated tensile reinforcement, the propagation of cracking is halted by stress re-distribution along the fibres. They also influence durability, impact resistance and tensile strengths of cement pastes. Fibres come in a variety of different sizes and materials. Some Common Materials for Fibres 1. Nylon 2. Polypropylene 3. Steel 4. Glass 5. Carbon 6. Cellulose 7. Kevlar Additional water, over and above the chemically bound water in a cement mix, migrates to become evaporable water. This moisture movement again forms continuities in the pore system and increases permeability, hence the relationship between water/cement and permeability (see Fig. 4). Long chain polymers can also control the movement of moisture by chemically binding water molecules so that fewer are free to migrate. POLYMER-MODIFIED CEMENT-BASED MATERIALS Polymers when used in cement-bound materials, come in two forms: i) spray dried polymer powders (ii) polymer dispersions Spray dried polymers are dispersion which are dried to form a water-re-dispersible powder which then acts in a similar way to a dispersion. Polymer dispersions as used in a cementitious environment, are a series of discrete "plastic" spheres in a solution of water. The spheres can come in a variety of different degrees of "hardness" and "stickiness", which impart different properties to the modified cement paste. As these spheres can be very small, of the order of 0.1m m in diameter, a stabilisation system is required in the water suspension in order to stop the polymer particles sticking together and forming large agglomerates. These usually take the form of ionic surfactants or colloids. As stated earlier in this paper, a grain of cement will only hydrate when it is totally surrounded by a membrane of water. However, the water membrane now contains polymer particles which gradually come together to form a film on the surface of the hydrates as the water becomes chemically bound, (it is generally accepted that it requires 10% polymer solids by weight of cement to reach this state). Once above minimum film-forming temperature, chemical bonding takes place between the particles (coalescing) to form coherent thermoplastic films coating the hydrates. Based upon this understanding, the mechanisms by which polymers enhance the performance of cement pastes can be explained. a) Increased Adhesion The introduction of "sticky" plastic spheres makes the cement paste more adhesive and cohesive. b) Increased Flexural Strength Relatively soft but strong plastic films give a greater degree of elasticity, but also act as reinforcement, giving increased flexural strength. It should, however, be noted that they are low modulus films and the implication that this has on the stress/strain relationship. c) Increased Abrasion Resistance A polymer rich surface has, essentially, a plastic coating which is better at resisting abrasion than an unmodified cement paste. d) Reduced Shrinkage The polymer spheres are small and can therefore block pores and capillaries and subsequently prevent water loss, reducing shrinkage. e) Improved Chemical Resistance The plastic coating on the surface has a much greater resistance to a variety of chemical attack than an unmodified cement paste. f) Reduced Permeability A similar mechanism to (d), capillary pores are blocked, reducing permeability. TYPES OF POLYMERS USED IN CEMENT-BOUND MATERIALS There are many different generic types of polymer dispersions and spray dried polymer powders now commercially available worldwide. However, the historic generations were as follows: First Generation: Polyvinyl Acetate (P.V.A.) Second Generation: Styrene Butadiene Rubber (S.B.R.) Acrylic Copolymers Third Generation: Styrene Acrylic Copolymers If the polymer molecule is made up from more than one "building block" (monomer), then in the case of two monomers it becomes a copolymer or, in the case of three, a terpolymer. As stated earlier, the polymer particles vary considerably in their physical properties, for example a S.B.R. being a synthetic rubber latex, is relatively soft and sticky, whereas a pure acrylic is hard and not very sticky. Therefore, the films which are formed also vary in their characteristics, necessitating the performance requirements to be matched for individual applications. CEMENT/POZZOLANIC MODIFIED MATERIALS Portland cement blended with one of the following:- 1. Microsilica 2. Pulverised Fuel Ash 3. Ground Granulated Blast Furnace Slag 4. Calcined China Clay can dramatically reduce the permeability and, hence, chloride ion diffusion of concrete. This group, when combined with the benefits of polymer modification, can offer good engineering solutions. THERMO-SETTING RESIN MODIFIED CEMENT-BASED MATERIALS Since this discovery in 1911, epoxides have enjoyed considerable success in a wide variety of applications, including surface coatings and adhesives. Their main attributes are toughness, low shrinkage on cure, high adhesion and good chemical resistance. The term "epoxy" or "epoxide" is applied to any resin containing a ring consisting of an oxygen atom attached to two carbon atoms on an adjacent position. The first and still the most important commercial epoxy resins are reaction products of Bisphenol A and Epichlorohydrin. In order to thermo-set the resin, a reaction with a curing agent is required which transforms them from low molecular weight materials to a highly cross-linked network. This network is composed of segments involving both epoxide and cross-linking agents. Research work has shown that this epoxy/curing agent adduct polymer when residing in a cementitious matrix, takes the form of short rod-like materials randomly interspersed within the hydrates. It is thought that these strands of cured epoxy polymer, act as reinforcement and help explain the increased strength properties of epoxy/cement composites. CEMENT-BOUND PROTECTIVE COATINGS Using the technology described earlier, it is now possible to produce cementitious coatings which have outstanding protective performance characteristics, whilst maintaining all the desirable features of being similar physically and chemically to base concrete and also, of even greater relevance, being totally "green" products as they are water-based. They can also be reinforced with a variety of meshes made to enhance the tensile strain capacity. By the incorporation of MCI’s existing in the vapour phase, sufficient active constituents can migrate and penetrate into the substrate and form protective films on the steel reinforcement. TYPICAL
MECHANICAL CHARACTERISTICS OF CEMENT-BOUND COATINGS
Compressive
Strength at 20¡ C: Flexural Strength: 11-14N/mm² Adhesive
Strength: 2N/mm² (concrete) Water
Permeability Coefficient: 1.43 x 10-16 m/sec Carbon Dioxide Gas Diffusion Resistance Coefficient: m CO2 = 2,600,000 Therefore
equivalent air thickness value R at 2mm Based
on Engelfried Technique an effective Oxygen Diffusion Coefficient: 4.42 x 10-5cm² s-1 Chloride
Ion Diffusion: No steady state flux of chloride ions after REFERENCES (1) Bakker, R.F.M., Illustration of Permeability and Porosity. Diffusion within and into Concrete. Paper presented at 13th Annual Convention of the Institute of Concrete Technology, University of Loughborough, 25-27th March 1985. Slough Institute of Concrete Technology, 1985. 21pp. (2) Lydon, F.D., Concrete Mix Design, Applied Science Publishers Limited, 1972. (3) Verbeck, G.J., and Vol. 3, Proceedings of the 5th Helmuth, R.H., International Symposium on the Chemistry of Cement, Tokyo 1968. (4) Powers, T.C., Copeland, Permeability of Portland Cement Paste ACI L.E., Hayes J.C., and Journal Proceedings, V.51. Mann, H.M., (5) Powers, T.C., Chemistry of Cement Proceedings, 4th International Symposium, Washington, 1960. (6) Robinson, H., Evaluation of Coatings as Carbonation Barriers, Construction Repair, February 1987, Vol. 1, No. 1. (7) Engelfried, R., Preventive Protection by Low Permeability Coatings, The Concrete Society One Day Conference, Permeability of Concrete and its Control, London, 12th December 1985. (8) Kostuck, J.A., Jones, T.R. New pozzolanic materials for the concrete industry. (9) Cortec Corporation Concrete Manual.
oooOOOooo |
||||||||||||||||||||||||||||||||||||||||||||||||||||
[About Cortec] [What's New] [Publications] [Products] [Contact Us] [Related Links] [International]
Cortec® and VpCI® are registered to Cortec Corporation