Prof. Dubravka Bjegović, Ph. D.,
University of Zagreb, Civil Engineering Faculty, Zagreb, Croatia
Jessica Jackson, Cortec Corporation, St. Paul,
MN, USA
Richard D. Stehly Ph.D., American Engineering
Testing, St. Paul, MN, USA
ABSTRACT
The bridge deck of the Randolph Avenue Bridge
in St. Paul, Minnesota, was rehabilitated in 1986. The rehabilitation
process included the application of an LSDC overlay to the two
eastbound traffic lanes and the application of an LSDC overlay
that included the corrosion-inhibiting admixture, MCI, to the
two westbound traffic lanes.
In this paper the results of corrosion rates,
electrochemical potential, chloride contamination level, concrete
resistivity, ambient temperature and relative humidity of both
the eastbound and westbound lanes on Randolph Avenue Bridge are
given. The purpose of this investigation was to determine if MCI
does indeed have a positive effect on the rate of corrosion, fourteen
years after the rehabilitation.
The westbound lanes, treated with corrosion inhibitor,
have lower half — cell potentials, lower corrosion rates
readings, lower chloride levels and higher concrete resistivity
than the eastbound lanes. This investigation shows positive effect
of migration corrosion inhibitor as the method for reinforced
concrete corrosion protection.
KEYWORDS: concrete, corrosion, corrosion-inhibiting
admixture, effectiveness, reinforcing steel
INTRODUCTION
One of the main causes of reinforced concrete
deterioration is the steel corrosion. The deterioration of reinforced
concrete structures is connected with enormous costs and therefore
the development of accurate corrosion protection methods is of
the greatest importance. One of the possible methods is the application
of migrating corrosion inhibitors (MCI).
MCI inhibitors are secondary electrolyte layer
mixed inhibitors. Substances dispersed or dissolved in the electrolyte-layer
cause electrolyte-layer inhibition. MCI possess appreciable saturated
vapor pressures under atmospheric conditions, thus allowing significant
vapor phase transport of the inhibitive substance (1). MCIs are
a mixed (anodic /cathodic) amino-carboxylate based inhibitor system.
The inhibition of cathodic process is achieved by incorporation
of one or more oxidizing radicals in an organic molecule. Inhibitor
molecules are hydrolyzed in the electrolyte and then adsorbed
on the metal surface. The nitrogen of the amine group is capable
of entering into a coordinate bond with metal thus enhancing the
adsorption process. Adsorption of cations increases the over potential
of metal ionization and slows down the corrosion. The monomolecular
film serves as a buffer to hold the pH at the interface in the
optimum range for corrosion resistance. MCI migrate or transport
through concrete to the rebar depending on the MCI type and application
method: MCI admixture type migrates to the reinforcement by diffusion
(both liquid and vapor) and MCI topical application type migrates
to the reinforcement first by capillary suction and than by diffusion
(both liquid and vapor) (2).
Bjegović et all (3) suggest an approach
to the migration corrosion inhibitor testing as the procedure
in four steps: step I - the electrochemical methods, as a preliminary
testing, step II - methods according the Japanese standard JIS
A 6205, as an accelerated test, step III - long-term laboratory
testing according to ASTM G 109 and step IV — field monitoring.
A lot of laboratory testing has been still done worldwide (4).
Only some field testing are in progress now.
One of such field testing was done on Randolph
Avenue Bridge. The corrosion rates, electrochemical potential,
chloride contamination level, concrete resistivity, ambient temperature
and relative humidity of both the eastbound and westbound lanes
on Randolph Avenue Bridgewere tested. The purpose of the investigation
was to determine if MCI does indeed have a positive effect on
the rate of corrosion, fourteen years after the rehabilitation.
In 1986, the deck of the bridge that carries
Randolph Avenue over I-35E in St. Paul, Minnesota, was rehabilitated.
The rehabilitation process included the application of a Low Slump
Dense Concrete (LSDC) overlay. Migrating corrosion inhibitor was
added to the LSDC at 1 lb/yd3 (0.6 kg/m3)
for the two westbound traffic lanes. Prior to overlay, the deck
was milled to a depth of 0.5 in (13 mm) and the areas of unsound
concrete were removed. The cavities from the removal of the unsound
concrete were filled with the overlay concrete.
Corrosion assessments were conducted on the eastbound
(control) and westbound (with MCI applied) travel lanes of the
structure on two occasions, June 1991 and August 1992. The assessments
included visual inspection, delamination survey, cover-depth survey,
chloride contents as a function of depth, corrosion potentials,
and estimates of corrosion current densities (icorr).
SHRP-S-658 contains all information from the 1991 and 1992 study.
In November 2000 new measurements has been done on the bridge.
These included Gecor 6 readings and copper/copper sulfate half-cell
potentials. A new chloride analysis was also taken at various
depths. This paper contain data obtained from 1985 to 2000.
EXPERIMENTAL PROCEDURE
Half-Cell Potentials
ASTM C 876 corrosion half-cell potentials were
measured for both the eastbound and westbound travel lanes with
a copper/copper sulfate electrode (CSE) before reconstruction
in 1985 and after from 1986 to 2000, five, six and fourteen years,
respectively after reconstruction; and with Gecor 6 in November
of 2000.and are given in Table 1.The results of the measurements
in 2000 are shown in Fig.1. According to ASTM: C876, the
results can be interpreted as follows in Table 2.
Table 1. East and Westbound Randolph St. Bridge Deck Half-Cell
Potential and Gecor 6 Readings
|
East and Westbound Randolph St. Bridge
Deck Half-Cell Potential and Gecor 6 Readings
|
| |
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
| |
|
|
Years Service
|
Average
|
Std.Dev.
|
Minimum
|
Maximum
|
%>350 mV
|
Testing Firm
|
| |
|
|
|
|
|
|
|
|
|
| |
|
Control
|
1985
|
256 mV
|
93
|
120 mV
|
620 mV
|
13
|
MN - DOT
|
| |
|
MCI
|
292mV
|
115
|
150 mV
|
650 mV
|
24
|
MN - DOT
|
| |
|
|
|
|
|
|
|
|
|
| |
|
Control
|
1986
|
100 mV
|
53
|
000 mV
|
270 mV
|
0
|
MN - DOT
|
| |
|
MCI
|
141 mV
|
48
|
030 mV
|
300 mV
|
0
|
MN - DOT
|
| |
|
|
|
|
|
|
|
|
|
| |
|
Control
|
1987
|
292 mV
|
59
|
157 mV
|
444 mV
|
15
|
MN - DOT
|
| |
|
MCI
|
302mV
|
52
|
180 mV
|
479 mV
|
18
|
MN - DOT
|
| |
|
|
|
|
|
|
|
|
|
| |
|
Control
|
1988
|
299 mV
|
49
|
204 mV
|
466 mV
|
12
|
MN - DOT
|
| |
|
MCI
|
270 mV
|
50
|
161 mV
|
485 mV
|
5
|
MN - DOT
|
| |
|
|
|
|
|
|
|
|
|
| |
|
Control
|
1989
|
177 mV
|
64
|
037 mV
|
527 mV
|
2
|
MN - DOT
|
| |
|
MCI
|
139 mV
|
56
|
009 mV
|
344 mV
|
0
|
MN - DOT
|
| |
|
|
|
|
|
|
|
|
|
| |
|
Control
|
1990
|
276 mV
|
73
|
162 mV
|
574 mV
|
15
|
MN - DOT
|
| |
|
MCI
|
238 mV
|
59
|
133 mV
|
512 mV
|
4
|
MN - DOT
|
| |
|
|
|
|
|
|
|
|
|
| |
|
Control
|
1991
|
149 mV
|
64
|
195 mV
|
237 mV
|
6
|
VA TECH
|
| |
|
MCI
|
179 mV
|
55
|
170 mV
|
235 mV
|
2,3
|
VA TECH
|
| |
|
|
|
|
|
|
|
|
|
| |
|
Control
|
1992
|
231 mV
|
64
|
217 mV
|
236 mV
|
NA
|
VA TECH
|
| |
|
MCI
|
184 mV
|
50
|
173 mV
|
236 mV
|
NA
|
VA TECH
|
| |
|
|
|
|
|
|
|
|
|
| |
|
Control
|
2000
|
248 mV
|
54
|
134 mV
|
407 mV
|
4
|
AET
|
| |
|
MCI
|
222 mV
|
55
|
145 mV
|
360 mV
|
1,4
|
AET
|
| |
|
Gecor 6 readings
|
|
|
|
|
|
|
| |
|
Control
|
2000
|
269 mV
|
67
|
175 mV
|
414 mV
|
12
|
AET
|
| |
|
MCI
|
209 mV
|
51
|
144 mV
|
333 mV
|
0
|
AET
|
Table 2. Criteria for corrosion potential measurement
|
Potential
|
Probability of Corrosion
|
|
>-200 mV
|
Less than 10%
|
|
-200 mV to -350 mV
|
50%
|
|
>-350 mV
|
Greater than 90%
|
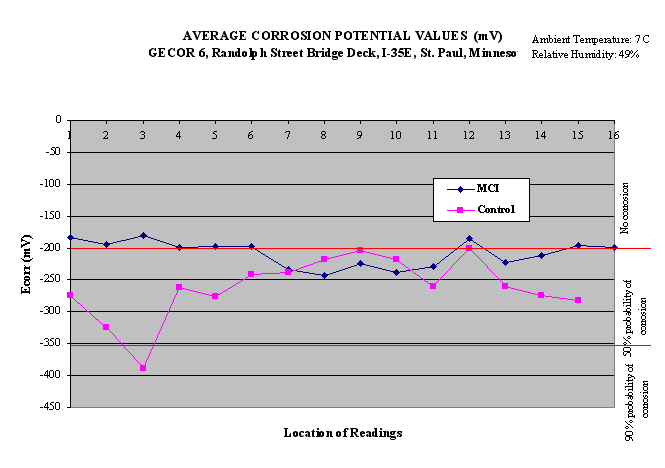
Fig.1. Corrosion potential measurements in 2000 year
Corrosion Current Measurements
Corrosion current density (icorr)
estimates were taken with the Gecor 6 device in November 2000.
These same estimates were taken in June 1991 and August 1992 using
a 3LP device. The icorr measurement using the 3LP device
is proportional to the corrosion rate through Faraday’s Law.
The Gecor 6 measures the corrosion rate of steel in concrete by
"polarization resistance" or "linear polarization"
techniques. This is a non-destructive technique that works by
applying a small current to the rebar and measuring the change
in the half-cell potential. Then the polarization resistance,
Rp, (the change in potential measured by Gecor 6),
is divided by the applied current. The Gecor 6 obtains the corrosion
rate, icorr, from the polarization resistance, Rp,
by means of the "Stearn and Geary" relationship:
icorr = B/Rp, where B = 26 mV (1)
The criteria for estimating the reinforcement’s condition
in relation to the measured value of the rate of corrosion have
been defined as follows in Table 3.
The results of the measurements obtained from 1991 to 2000 are
shown in Table 4 and only for 2000 but for different location
are shown in Fig.2.
Table 3. Criteria for corrosion rate
|
Icorr
|
Intensity of Corrosion
|
|
<0.1 to 0.2 ľA/cm2
|
Passive condition
|
|
0.2 to 0.5 ľA/cm2
|
Low to moderate corrosion
|
|
0.5 to 1.0 ľA/cm2
|
Moderate to high corrosion
|
|
>1.0 ľA/cm2
|
High corrosion rate
|
Table 4. Corrosion rates measurements from 1991 to 2000.
| |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Statistical Parameter
|
|
|
Icorr (mA/ft2)
|
|
|
|
| |
Eastbound, LSDC Control
|
|
Westbound,LSDC/Cortec MCI 2000
|
| |
1991*
|
1992*
|
2000**
|
|
1991*
|
1992*
|
2000**
|
|
|
Mean
|
1,53
|
0,74
|
0,02
|
|
1,83
|
0,51
|
0,012
|
|
|
Standard Deviation
|
0,45
|
0,2
|
0,01
|
|
1,44
|
0,18
|
0,008
|
|
|
Number Observati.
|
11
|
12
|
32
|
|
11
|
6
|
41
|
|
| |
|
|
|
|
|
|
|
|
|
*Taken with 3LP meter
|
**Taken with Gecor 6
|
|
|
|
|
|
1m A/cm2
= 0.929 mA/ft2
|
|
|
|
|
|
|
|
Concrete Resistivity Measurements
Concrete resistivity measurements were also taken using Gecor
6 device and concrete resistivity was calculated by means of the
formula:
Resistivity = 2 * R * D, where (2)
R = resistance by the "iR drop" from a pulse between
the sensor counter-electrode and the rebar network
D = counter-electrode diameter of the sensor
The value of the concrete’s resistance is used as an additional
parameter for the interpretation of the rate of corrosion. The
interpretation of the results is possible according the criteria
by Andrade et all (5,6) given in Table 5. The results of the measurements
obtained only in 2000 year are shown in Fig.3.
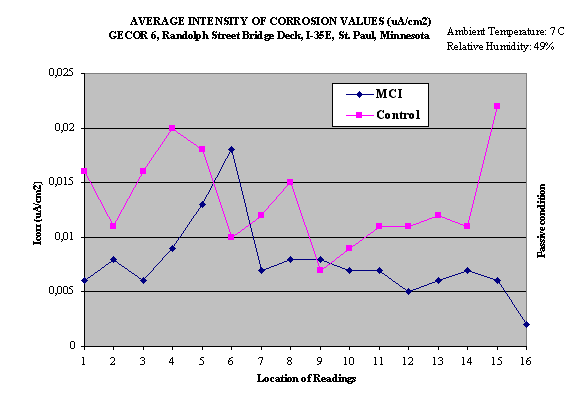
Fig.2. Corrosion rate measurements in 2000 year
Table 5. Criteria for Concrete Resistivity
|
Resistivity
|
Corrosion Rate
|
|
>100 to 200 kW
· cm
|
Very low, even with high chloride and carbonation
|
|
50 to 100 kW
· cm
|
Low
|
|
10 to 50 kW
· cm
|
Moderate to high where steel is active
|
|
<10 kW
· cm
|
Resistivity is not the parameter controlling
corrosion rate
|
A graph of the corrosion current and resistance
(Icorr-R) results should be used so as to avoid wasting results.
Andrade et al (6) recommend that criteria for the estimation of
the intensity of corrosion should be used as shown in Fig. 4 in
which the real results from the Randolph Avenue Bridge investigation
are also given.
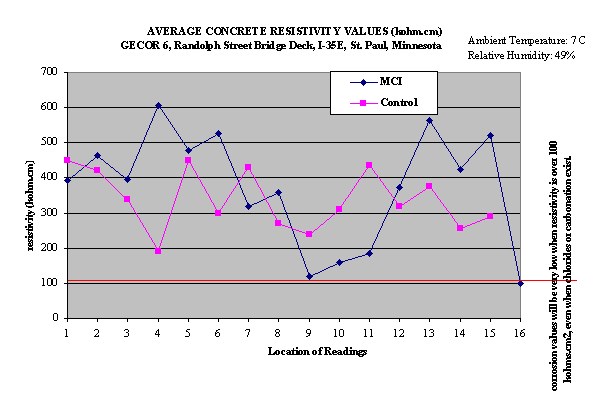
Fig.3. Concrete resistivity measurements in 2000 year
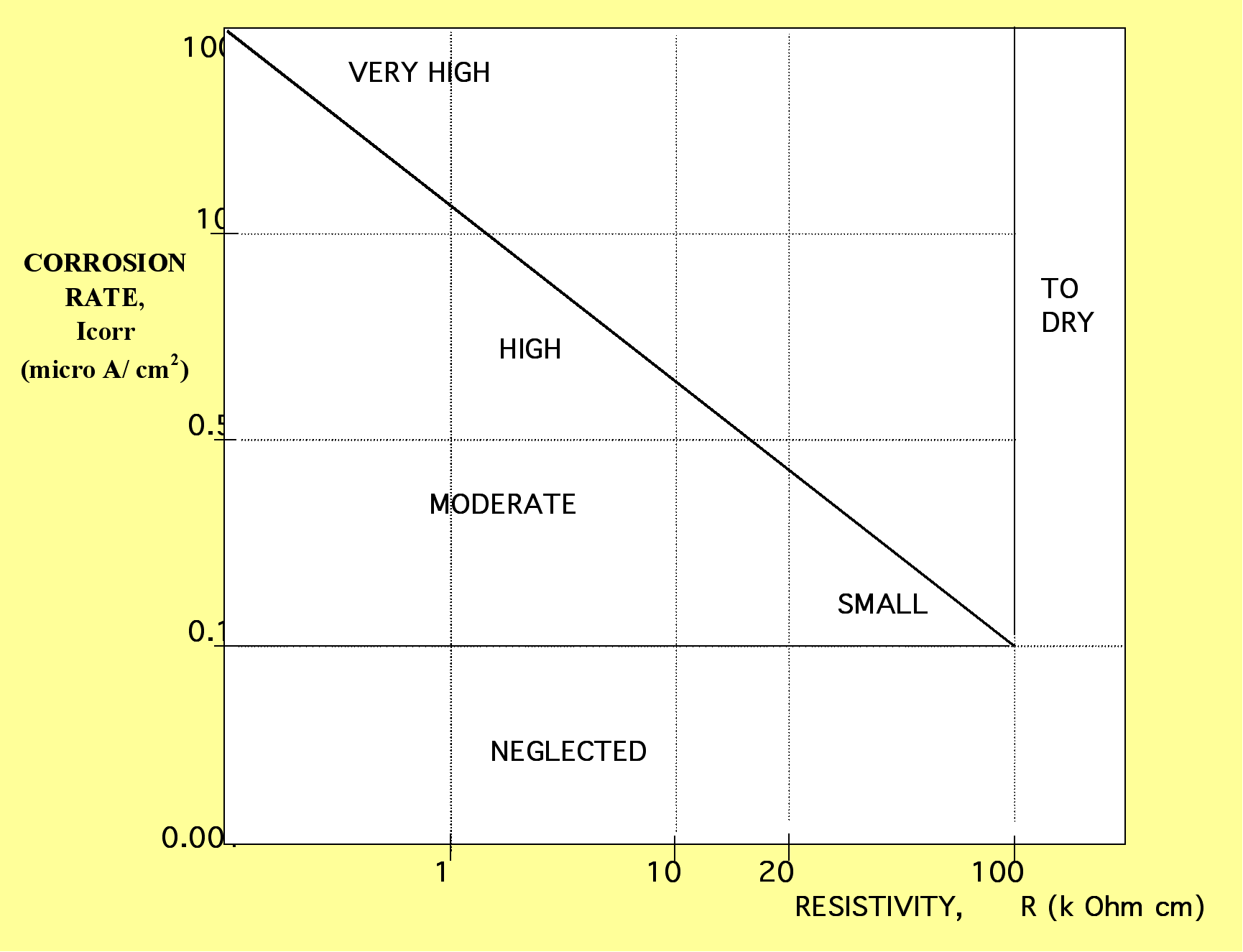
Fig.4. Corrosion rate and resistivity relationship
When the research results of a structure are
described in a graph such as in Fig. 4., it should be expected
that, if corrosion is active, the points of the graph follow the
main line and should be in harmony with the degrees of saturation
of the concrete structure.
Chloride Contamination Levels
Powdered concrete samples for chloride analysis
were taken at mean depths of 1.0, 2.0, and 3.0 in (25, 51, and
76 mm) from 3 different locations on each side of the bridge in
1991, 1992 and 2000. Samples were taken using a rotary impact
type drill with a _" sized bit. Three-gram samples that passed
through a #20 sieve were obtained from each depth. The powder
was then mixed with 20 ml of digestion solution for a total of
3 minutes and then 80 ml of stabilizing solution was added. A
calibrated electrode coupled to an Orion Model 720-pH/ISE meter
was then immersed in the solution, and the chloride-ion concentration
was recorded. This method was consistent with the AASHTO: T260
procedure. The standard deviation for this chloride test was determined
by testing the six pulverized concrete Quality Assurance (QA)
samples of known chloride content. Each QA sample was tested five
times. The results are given in Tables 6 and 7.
Table 6. Chloride — Ion Content (lbs/yd3) for
Eastbound lanes, LSDC control
(1991&1992 data: Virginia Technology; 2000 data: American
Engineering Testing)
|
Depth
|
1991
|
|
1992
|
|
2000
|
|
|
(in)*
|
mean
|
s x
|
mean
|
s x
|
mean
|
s x
|
|
0.5
|
10.7
|
2.4
|
13.7
|
3.4
|
-
|
-
|
|
1.0
|
4.7
|
2.3
|
5.7
|
2.9
|
17.2
|
1.8
|
|
1.5
|
2.2
|
1.8
|
3.0
|
1.8
|
-
|
-
|
|
2.0
|
2.7
|
0.9
|
4.0
|
2.0
|
6.2
|
0.6
|
|
2.5
|
2.3
|
1.4
|
3.0
|
2.0
|
-
|
-
|
|
3.0
|
1.4
|
1.6
|
1.9
|
1.8
|
2.4
|
0.4
|
|
3.5
|
0.3
|
0.4
|
-
|
-
|
-
|
-
|
(1991&1992 data: Virginia Technology; 2000 data: American
Engineering Testing)
*1 in = 2,54 cm
In 1991 chloride content was 40% less than the
control. The chloride levels increased at all levels in 1992 and
2000.
According to the LIFE-365 Service Life Prediction
model (7), and on the basis of the data from chloride content
in concrete overlay, Fig. 5. and the LSDC mix design for Randolph
Avenue Bridge Overlay, Table 8. the calculation for time until
chloride threshold is reached was done and given in Table 9.
Table 7. Chloride — Ion Content (lbs/yd3) for
Westbound lanes, LSDC/ MCI 2000
(1991&1992 data: Virginia Technology; 2000 data: American
Engineering Testing)
|
Depth
|
1991
|
|
1992
|
|
2000
|
|
|
(in)*
|
mean
|
s x
|
mean
|
s x
|
mean
|
s x
|
|
0.5
|
6.0
|
2.2
|
9.6
|
1.8
|
-
|
-
|
|
1.0
|
1.1
|
1.3
|
3.3
|
1.5
|
11.7
|
0.9
|
|
1.5
|
0
|
0.1
|
1.2
|
1.3
|
-
|
-
|
|
2.0
|
0
|
0
|
1.0
|
1.1
|
1.6
|
0.5
|
|
2.5
|
0.4
|
0.6
|
1.3
|
0.9
|
-
|
-
|
|
3.0
|
1.0
|
0.9
|
2.5
|
1.0
|
1.3
|
0.2
|
|
3.5
|
0.9
|
0.8
|
-
|
-
|
-
|
-
|
(1991&1992 data: Virginia Technology; 2000 data: American
Engineering Testing)
*1 in = 2,54 cm
a) Eastbound lanes, LSDC Control b) Westbound lanes,
LSDC/ MCI 2000
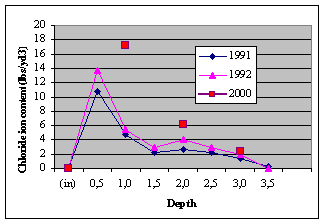
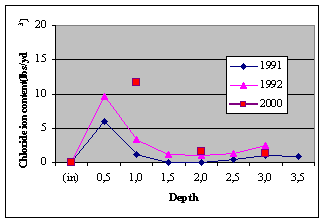
Fig. 5. Chloride contamination levels
|
Table 8. LSDC Mix Design for Randolph Avenue Bridge Overlay
|
|
Component
|
|
|
|
|
SSD lbs/yd3
|
|
Type I Cement
|
|
|
|
|
836
|
|
Water
|
|
|
|
|
270
|
|
Design w/c
|
|
|
|
|
0,32
|
|
Coarse Aggregate
|
|
|
|
|
1385
|
|
Fine Aggregate
|
|
|
|
|
1374
|
|
Water Reducing Admixture, PDA25YL
|
|
|
|
|
32.0 oz
|
|
AEA, Protex
|
|
|
|
|
9.3 oz
|
|
Note: 1 lb/yd3 = 0.59 kg/m3, 1 oz
= 28.4 g
|
|
|
|
|
|
Table 9. Calculation for time until chloride threshold is reached
| |
|
|
Control
|
|
|
|
|
|
|
|
2.4 lbs/yd3 chlorides at 3" 14 years after overlay was
placed. 836 lbs/yd3 of cement in mix design.
|
|
|
2.4 / 836 * 100 = 0.29% Cl- by weight of cement in 14 years.
|
|
|
|
|
|
0.29% / 14 = 0.02% Cl- per year * 19.5 years = 0.40% Chlorides
= chloride threshold
|
|
|
|
1986 + 19.5 = 2005.5 (year we expect chloride threshold
to be reached in control concrete)
|
|
|
|
MCI
|
|
|
|
|
|
|
|
1.3 lbs/yd3 chlorides at 3" 14 years after overlay was
placed. 836 lbs/yd3 of cement in mix design.
|
|
|
1.3 / 836 * 100 = 0.16% Cl- by weight of cement in 14 years.
|
|
|
|
|
|
0.16% / 14 = 0.011% Cl- per year * 36 years = 0.40% Chlorides
= chloride threshold
|
|
|
|
1986 + 36 = 2022 (year we expect chloride threshold to
be reached in MCI treated concrete)
|
|
|
|
(*uses 0.40% of chlorides by weight of cementious materials.)
|
|
|
|
|
Conclusion
The LSDC (Low Slump Dense Concrete) has done
an excellent job at reducing the corrosion rate of the steel on
both the control and MCI treated sides by reducing the diffusion
of chlorides into the concrete. The conditions of the concrete
on the bridge were wet and cool, taken after a period of cold
weather and rain for several days. This was because the readings
with the Gecor 6 are more accurate regarding the corrosion rate
in wet conditions. While the LSDC has done an excellent job at
protecting the reinforcing steel from corrosion on both sides
of the bridge, a difference between readings taken on the control
and MCI treated sides can still be seen:
- The average copper/copper sulfate potential value for rebar
embedded in the MCI treated overlay was 208.9 mV, 22% less than
the average for the control overlay at 269.6 mV. Also, 12% of
the readings taken on the control overlay indicated corrosion,
while none did on the MCI treated side.
- Rebar on the MCI 2000 treated side had very low corrosion
currents, an average of 0.013 m
A/cm2, approximately 40% below readings taken on
control side (average of 0.022 m
A/cm2).
- The reduced chloride diffusion can be seen when looking at
the data from American Engineering Testing. After fourteen years,
the chloride concentration at three inches (76 mm) of depth
(near the rebar) is 1.42 kg/m3 on the control side
and 0.77 kg/m3 on the MCI treated side.
- According to the LIFE-365 Service Life Prediction model, the
chloride threshold (Ct) is 0.05%, this value
is commonly used for service-life prediction purposes and is
close to a value of 0.40% chloride based on the mass of cementitious
materials for a typical structural concrete mix. This is the
level of chlorides at which corrosion of the reinforcing steel
is initiated. It is estimated that the control will reach 0.05%
of chlorides at the rebar in 19.5 years from the application
of the overlay, or in the year 2005, if the chloride level continues
to increase at its current rate. The MCI 2000 treated side is
estimated to reach 0.05% of chlorides in 36 years from the application
of the overlay, or in the year 2022.
REFERENCES
- Miksic, B.A., (1983), Use of Vapor Phase Inhibitors for Corrosion
Protection of Metal Products, NACE Corrosion 83, Paper No. 308,
Anaheim, California.
- Bjegović, D., Sipos, L., et al., (1994), Diffusion of
the MCI 2020 and 2000 Corrosion Inhibitors into Concrete, International
Conference on Corrosion and Corrosion Protection of Steel in
Concrete, Sheffield, 865-877.
- Bjegović, D., Mik_ić, B., Stehly, R., (2000), Test
Protocols for Migrating Corrosion Inhibitors (MCITM)
in Reinforced Concrete, in Emerging Trends in Corrosion Control
— Evaluation, Monitoring, Solutions, published by:
Akademia Books International, New Delhi, 3-18.
- COST 521: Corrosion of Steel in Reinforced Concrete Structures,
Prevention — Monitoring — Maintenance, Final Report
2002, edited by Romain Weydert, published at IST — Luxembourg
University of Applied Sciences, pp 254.
- Andrade, C., Alonso, M.C., Gonzalez, J.A., "An Initial
Effort to Use the Corrosion Rate Measurements for Estimating
Rebar Durability", Corrosion Rates of Steel in Concrete,
ASTM STP 1065.
- Andrade, C., Fullea, J., Alonso, C., "The Use of the
Graph Corrosion Rate-Resistivity in the Measurement of Corrosion
Current", International Workshop MESINA, Instituto Eduardo
Torroja, Madrid, Spain, 1999.
- Thomas, M.D.A. and Bentz, E.C., Life-365 Computer Program
for Predicting the Service Life and Life-Cycle Costs of Reinforced
Concrete Exposed to Chlorides. University of Toronto, Toronto,
Canada, April 21, 2000, pp. 9.

